Stoichiometry
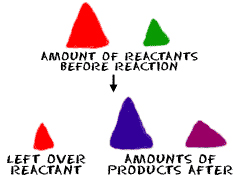 Let's start with how to say this word. Five syllables. STOY-KEE-AHM-EH-TREE. It's a big word that describes a simple idea. Stoichiometry is the part of chemistry that studies amounts of substances that are involved in reactions. You might be looking at the amounts of substances before the reaction. You might be looking at the amount of material that is produced by the reaction. Stoichiometry is all about amounts.
Let's start with how to say this word. Five syllables. STOY-KEE-AHM-EH-TREE. It's a big word that describes a simple idea. Stoichiometry is the part of chemistry that studies amounts of substances that are involved in reactions. You might be looking at the amounts of substances before the reaction. You might be looking at the amount of material that is produced by the reaction. Stoichiometry is all about amounts.
Underline a definition of stoichiometry given above.
All reactions are dependent on how much stuff you have. Stoichiometry helps you figure out how much of a compound you will need or maybe how much you started with. We want to take the time to explain that reactions are dependent upon the compounds involved and how much of each compound is needed.
Underline what reactions are dependent upon as outlined above.
 What do you measure? It could be anything. When you're doing problems in stoichiometry, you might look at...
What do you measure? It could be anything. When you're doing problems in stoichiometry, you might look at...
- Mass of Reactants (chemicals before the reaction)
- Mass of Products (chemicals after the reaction)
- Chemical Equations
- Molecular Weights of Reactants and Products
- Formulas of Various Compounds
Q: Reactions start with products and create reactants.
 True
True
 False
False
A: False. Reactions start with reactants and finish with products. A simple reaction might combine two hydrogen molecules (H2) with one oxygen molecule (O2). The product would be water (H2O). There are millions of more complex reactions.
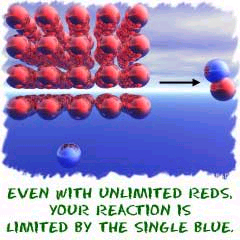
Now an example. Let's start with something simple like Sodium chloride (NaCl). You start with two ions and wind up with an ionic compound (NaCl). When you look at the equation, you see it takes one atom of sodium (Na) to combine with one atom of chlorine (Cl) to make the salt. When you use stoichiometry, you can determine amounts of substances needed to fulfill the requirements of the reaction. Stoichiometry will tell you that if you have ten million atoms of sodium (Na) and only one atom of chlorine (Cl) you can only make one molecule of sodium chloride (NaCl). Nothing you can do will change that. Like this:
10,000,000 Na + 1 Cl --> NaCl + 9,999,999 Na
Underline what stoichiometry enables the scientist to determine to fulfill the requirements of a reaction.
 Let's bump it up a level. When you mix hydrogen gas (H2) and oxygen gas (O2), nothing much happens. When you add a spark to the mixture, all of the molecules combine and eventually form water (H2O). What does stoichiometry look at here? First, look at the equation. Four hydrogen (H) atoms and two oxygen (O) atoms are on each side of the equation. It's an important idea to see that you need twice as many hydrogen atoms as you do oxygen atoms. The number of atoms you need will help you figure out how much of each substance you will need to make the reaction happen.
Let's bump it up a level. When you mix hydrogen gas (H2) and oxygen gas (O2), nothing much happens. When you add a spark to the mixture, all of the molecules combine and eventually form water (H2O). What does stoichiometry look at here? First, look at the equation. Four hydrogen (H) atoms and two oxygen (O) atoms are on each side of the equation. It's an important idea to see that you need twice as many hydrogen atoms as you do oxygen atoms. The number of atoms you need will help you figure out how much of each substance you will need to make the reaction happen.
Q: Stoichiometry is a long word that describes--
 The number of atoms in the reactants equaling the number of atoms in the products.
The number of atoms in the reactants equaling the number of atoms in the products.
 The process of breaking down waxes into fatty acids.
The process of breaking down waxes into fatty acids.
 The process of electrons jumping from one orbital to another.
The process of electrons jumping from one orbital to another.
 None of the Above
None of the Above
A: Stoichiometry is the term used to describe the measurement of the number of atoms involved in a chemical reaction. For a reaction to occur, the number of atoms of reactants needs to equal the number of atoms of products. A reaction could not proceed if you have one hydrogen molecule (H2) and one oxygen (O2) molecule. Stoichiometry tells us that we need two hydrogen molecules to have the reaction proceed to create two water molecules (H2O).
adapted from Rader's Chem4kids.com
 Let's start with how to say this word. Five syllables. STOY-KEE-AHM-EH-TREE. It's a big word that describes a simple idea. Stoichiometry is the part of chemistry that studies amounts of substances that are involved in reactions. You might be looking at the amounts of substances before the reaction. You might be looking at the amount of material that is produced by the reaction. Stoichiometry is all about amounts.
Let's start with how to say this word. Five syllables. STOY-KEE-AHM-EH-TREE. It's a big word that describes a simple idea. Stoichiometry is the part of chemistry that studies amounts of substances that are involved in reactions. You might be looking at the amounts of substances before the reaction. You might be looking at the amount of material that is produced by the reaction. Stoichiometry is all about amounts.  What do you measure? It could be anything. When you're doing problems in stoichiometry, you might look at...
What do you measure? It could be anything. When you're doing problems in stoichiometry, you might look at...  Let's bump it up a level. When you mix hydrogen gas (H2) and oxygen gas (O2), nothing much happens. When you add a spark to the mixture, all of the molecules combine and eventually form water (H2O). What does stoichiometry look at here? First, look at the equation. Four hydrogen (H) atoms and two oxygen (O) atoms are on each side of the equation. It's an important idea to see that you need twice as many hydrogen atoms as you do oxygen atoms. The number of atoms you need will help you figure out how much of each substance you will need to make the reaction happen.
Let's bump it up a level. When you mix hydrogen gas (H2) and oxygen gas (O2), nothing much happens. When you add a spark to the mixture, all of the molecules combine and eventually form water (H2O). What does stoichiometry look at here? First, look at the equation. Four hydrogen (H) atoms and two oxygen (O) atoms are on each side of the equation. It's an important idea to see that you need twice as many hydrogen atoms as you do oxygen atoms. The number of atoms you need will help you figure out how much of each substance you will need to make the reaction happen.